33: A Thin Line
Episode 33: A Thin Line
Welcome back! And once again, thanks for your patience. My semester at GVSU has finally ended- no more exams, no more homework for another four months. And that means, more time for the podcast.
Before we dive back in, I have a plug to make. A few months ago, I was interviewed by another science podcast: Common Descent. They cover more recent paleontology, everything from dinosaurs to dragonflies, stuff we won’t cover on this show. It’s good stuff- you should really check it out if you’re hungry for more familiar fossils.
My interview is being released today, May 1, 2024. So after you’ve finished this episode, check out The Common Descent Podcast to hear my interview with David and Will. You’ll learn how I make this show, and how I got my start in science education.
On the other hand, if you’re a new listener who liked the interview, welcome aboard! To start at the beginning of Earth History, January 1st on the Earth Calendar, I’d recommend Episode 4. But, if you’re a fossil nerd, you’re in luck. Today, we’ll meet some strange, controversial rocks that might be the oldest fossils on Earth, or a red herring.
Last season, we spent eight episodes on the origins of life, from carbon in the stars to the first cells. We finished with the ancestor of all life on Earth, an extinct microbe called LUCA. However, there’s a dirty little secret: there are no fossils of LUCA. The only clues LUCA left behind are the genes in the DNA of every living thing. These genes have been copied and edited over four billion years, like a tall tale, or a whale song. But poetic as that is, Bedrock is a geology podcast, and we want something tangible, a rock we can point to and say, that’s the oldest fossil on Earth. To be fair, the early Earth doesn’t have a lot to work with.
In Season 1, January on our Earth Calendar, there were no rocks at all, just a few scattered crystals. Geologists are scouring these tiny cystals for any trace of life, but there’s no fossils yet. This season, we met Earth’s oldest rocks, February on the Calendar, 4 billion years old. But these rocks were forged in magma, much too hot for life. Again, no fossils here.
And now we find ourselves on March 3rd of the Calendar, 3.8 billion years ago. We finally have rocks that could preserve fossils, rocks that formed on the bottom of the sea. These rocks are found in northern Quebec, a site called Nuvvuagittuq. Today, Nuvvuagittuq is a cold tundra shoreline, but 3.8 billion years ago, it was a hot undersea volcano, belching up lava and boiling water.
These volcanic waters are rich in iron, a delicious broth for ancient microbes to sip. In the modern day, bacteria take iron-rich waters in swamps and make rusty bog iron. At Nuvvuagittuq, ancient microbes turned entire seafloors to rust, making beautiful banded iron formations or BIFs for short, which we met last week.
But again, if life was present, where are the bodies, where are the fossils?
Today, we will search the iron formations of Nuvvuagittuq for any signs of ancient life. Some folks say Earth’s oldest fossils are here, 3.8 billion years ago in Canada. Others aren’t so sure. I’ll lay out the evidence and you can be the jury. Let the debates begin!
Part 1: From Grey to Red
Before we look at the fossil candidates, let’s start by reviewing banded iron formations or BIFs from last episode- what they are, and how they’re made.
Banded iron formations formed on ancient seafloors, and are the backbone of the iron and steel industries. On this show, BIFs will be our companions until the last season, an empire of ancient iron that rose and fell. Unlike most rocks we’ll meet, it’s impossible to make BIFs today- Earth’s chemistry has changed too much.
If you find a BIF, you’ll see beautiful tiger stripes of grey and red. How did these two colors form? The grey layers formed underwater when dissolved, invisible iron met dissolved, invisible oxygen, making very visible steely minerals like magnetite and hematite. One way to pair iron and oxygen is with bacteria, and BIFs are often viewed as secondhand evidence for ancient life. Some bacteria make their own oxygen, just like plants, and let iron figure out the rest on its’ own. Other bacteria are more hands-on; they harvest energy directly from iron, making minerals as a by-product. Both recipes can make BIFs, but today we’ll focus on the second one. These micro-managing bacteria are called iron oxidizers.
OK, so if the gray layers inside BIF were made by bacteria, you would expect to find fossils there. But the candidates we’ll meet today are in the red layers that we ignored last time. So what’s happening in the other half of BIF, the red half?
The red layers are very different than their gray iron neighbors. This new material will be a frequent guest on the show. It’s time to meet chert, that’s c-h-e-r-t with a hard “ch”, not a shirt you’d wear.
Chert isn’t a household name. but it is extremely common. In rock shops, chert and its’ cousins go by a thousand other names you might recognize: flint, jasper, agate, and onyx. They all have the same major ingredients: the elements silicon and oxygen. Pure chert is usually white or gray, but just a pinch of other elements can make a rainbow of different colors. A dash of iron will turn chert blood red, just like we see inside BIFs.
Chert is a sedimentary rock, so it needs water to form. Many modern cherts are made in the ocean, when tiny plankton are buried on the seafloor. But these plankton weren’t around in the Archean. In fact, we’ll never see them on the show. But chert doesn’t need life to form- there are plenty of other recipes. Any water that is stuffed with silicon can make chert on its’ own. The best examples are hot volcanic springs like Yellowstone National Park. If you look around geysers like Old Faithful, you’ll see gray and white crusty rocks, and if you’re lucky, some are stained orange and red with iron.
Which brings us back to Nuvvuagittuq, 3.8 billion years ago. These ancient cherts formed in hot springs deep in the ocean, rich in silicon and iron. Let’s put all the pieces together and tell a story of red and gray.
We’re sitting in our imaginary submarine at the bottom of the ancient sea. In the darkness, our spotlights show a towering black chimney, surrounded by rusty red chert. Things have been quiet for a while. Then, suddenly, a dark cloud bellows up the chimney, pumping in much more iron than before. Bacteria quickly go to town, feasting on this bonanza and raining down iron minerals, which will become the gray layers in BIF. The scene settles down, and a new red layer of chert is formed. Repeat this process over many years, red chert and gray iron, red chert and gray iron, and you’ll make a banded iron formation.
Bacteria were here the whole time, through feast and famine. With that in mind, let’s see if they left any fossils behind at Nuvvuagittuq in Canada.
Part 2: Breaking News
The year is 2016. 2016?! That’s only eight years ago! Most of our research stories happened decades or centuries ago, but this debate is fresh. Nuvvuagittuq is really on the cutting edge of science. Anyways, back to the story.
The year is 2016. The place: University College London.
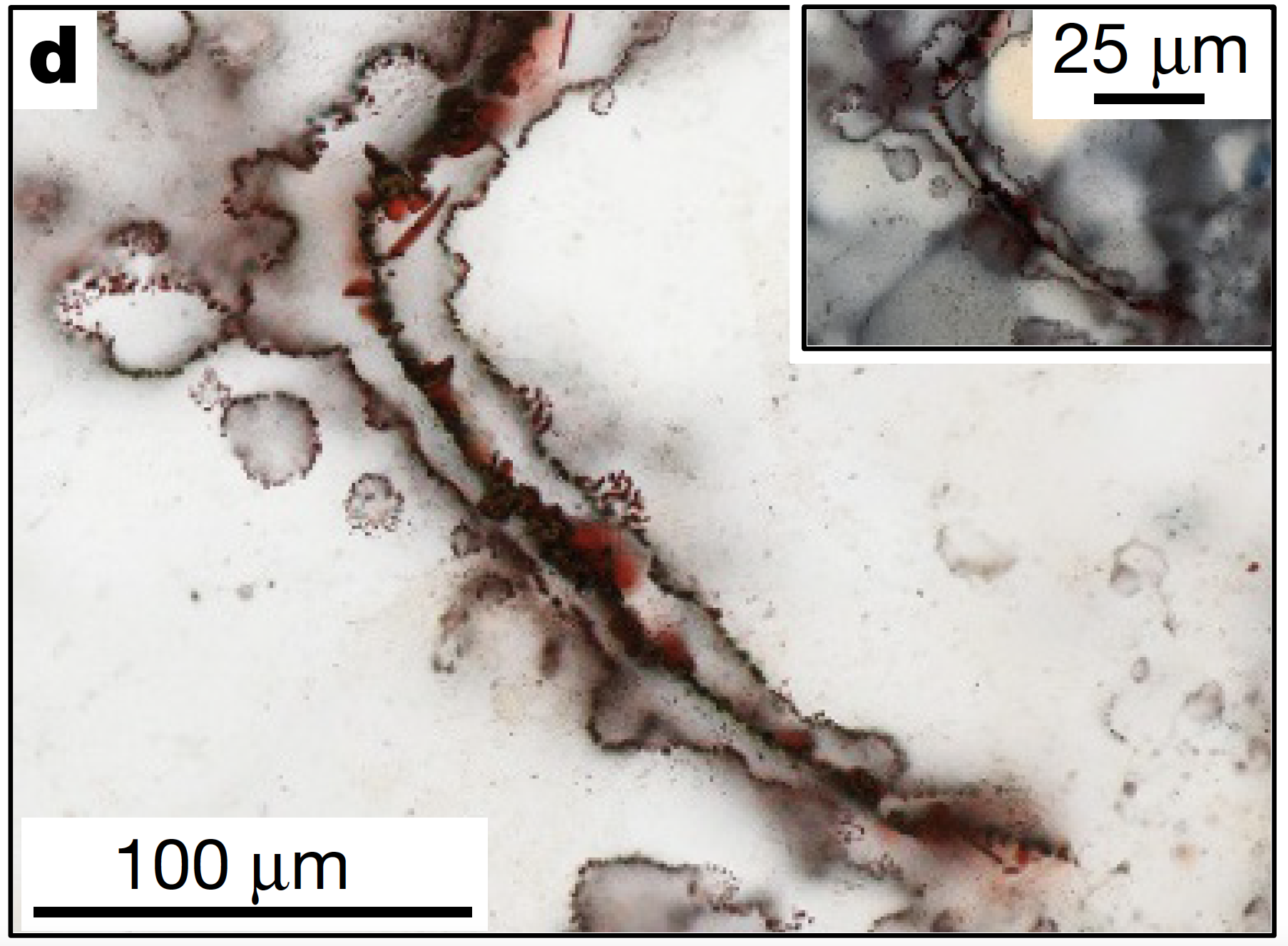
Inside a laboratory, a PhD student named Matthew Dodd is sitting at a microscope. He’s looking down on a very thin slice of rusty red chert, a piece of banded iron formation from Nuvvuagittuq. The slice is half the width of a human hair- so thin that light passes through it, like a tiny stained-glass window.
But inside this window, there are some microscopic specks that aren’t transparent- they stick out like a sore thumb. Dodd is using the microscope to get a better look, and what he sees is incredible. I’ve put some images up on our website: bedrockpodcast.com, so you can follow along.
What he saw were thin red threads of rust, frozen in a sea of clear crystal. Some twisted and coiled like dead worms, others formed branching patterns like sticks on a tree. A few even looked like blood-red soda straws- straight as an arrow and hollow on the inside. All these features were thinner than the average human hair- less than 30 microns wide.
What the heck were these things?
To answer that question, Dodd began looking for modern analogues, places on Earth that most closely resemble ancient Nuvvuagittuq. He was looking for deep sea volcanos with hot springs belching out rusty iron, just like we saw last section. Such places can still be found in the Ring of Fire around the Pacific Ocean, from Chile to Hawaii to Indonesia. Dodd didn’t visit these places himself, but he read papers from those who had, searching for modern images that matched his old, old rocks.
And he found some close matches! Around these deep vents, other researchers had found thin rusty threads and tubes. They were the same size and shape as Dodd’s samples, 3.8 billion years earlier. And the modern threads were made by life.
Last episode, we met a special group of bacteria called iron oxidizers. To survive, these bacteria don’t eat other critters like animals do, and they don’t need sunlight like plants do. Instead, they get their energy by taking invisible iron dissolved in water and turning it into rust. Now that rust has to go somewhere, and it often coats the bacteria themselves. Iron oxidizers can take many shapes and sizes, but most are shaped like long, thin, threads or tubes.
In short, modern iron oxidizing bacteria make rusty threads just like the ones in Canada 3.8 billion years ago. If you’re new to the show, that might sound like a no-brainer, case closed. But if you’ve been around since Season 1, this story might sound familiar.
In Episode 19, we met a special asteroid, a piece of Mars that crashed into Antarctica. That asteroid also had thin threads that also looked a lot like fossil bacteria. The asteroid was hailed as proof of life on Mars, but on further examination, the story began to fall apart. Every feature on the meteorite could be explained without needing life at all.
The search for Earth’s earliest life is surprisingly similar to the search for aliens. Finding a weird rock is not enough. Even finding a thread that looks just like bacteria is not enough. To prove that something is a fossil, you need to prove that nothing else could have made it.
As Carl Sagan said, “Extraordinary claims require extraordinary evidence.”
Back in the lab, Dodd and his co-authors knew this idea very well. Iron can form rusty threads without needing life at all. Sometimes rust forms around long, thin crystals. Sometimes rust is stretched out like silly putty, as it’s buried thousands of kilometers deep.
We’ll meet some other alternatives in just a second.
In 2016, the research team compared all their ancient rusty threads with all the modern rusty examples, living or not. They found only bacteria explained the thread patterns 3.8 billion years ago, bacteria were the only things that matched.
In short, these things had to be Earth’s oldest fossils.
In 2017, Dodd and company published their research in the journal Nature, the highest place a geologist can go.
Let’s fast-forward and see how that idea has held up over time.
Part 3: The Garden of Iron
Two years later, the rebuttals began.
A few folks say flat-out that the rusty threads are not fossils, period. More folks take a middle path, not completely dismissing the 2017 paper, but noting there are alternatives. So what else could these 3.8 billion year old tubes be, if not fossils?
Most arguments focus on a single candidate, something called a “chemical garden”.
Don’t worry if you’ve never heard that phrase before. Chemical gardens have been made since the 1600s, but have only become popular in the last few decades.
In fact, you can buy a chemical garden kit online- they’re popular home-made experiments for families with budding young scientists. If you don’t want to drop $30-50, you can just type in “chemical garden” into Youtube. I’ll post a video on our website.
An excellent video showing chemical garden growth. Warning: the bass in this video is a bit overbearing
What you’ll see is something beautiful. To start, you need to mix silicon into a glass of water. Then, you place a brightly colored crystal at the bottom of the mix and wait. Over time, that crystal will grow upwards into thin threads of blue, yellow or red depending on the mineral.
What’s happening here? To make a long chemical story short, the colorful crystal is reacting to the surrounding fluid, making a small pocket of acid. That acid bubble is quickly coated with tiny crystals like sprinkles on a cookie. But eventually pressure inside builds up, and the acid makes a break for the surface, only to be trapped once again by the sprinkles.
Repeat this process over time, and you’ll form a thin tube of colorful crystals. Not any crystals will do, but iron minerals are usually a good candidate.
Hopefully you can see where I’m going with this: the ingredients to make a chemical garden are the same ingredients inside our banded iron formations: lots of silicon, lots of iron.
In 2019, Sean McMahon at the University of Edinburgh made his own chemical gardens from iron. The thin, rusty threads he made in the lab were very similar to the ancient threads Dodd found from Nuvvuagittuq. No life required.
Also in 2019, similar features were also found Karen Johannessen from the University of Bergen, Norway. Her team found rusty iron tubes made with and without bacteria at the same location: at the bottom of the sea, next to volcanic vents just like ancient Nuvvuagittuq. Some needed life, but others didn’t.
In 2022, University College London struck back. They went back to Nuvvuagittuq and collected even more samples. They found new patterns of tiny threads that had never been made by a chemical garden before, that were probably fossils.
But even here, the London team noted that the chemical garden field is still young, and it’s possible that someone, somewhere will make a pattern just like these “fossils”, without any life required. It feels like they’re bracing themselves for the next round of debate.
So where does that leave us?
There are even more arguments for and against that I haven’t touched, that we don’t have time for. They all boil down to this:
The thin rusty threads at Nuvvuagittuq are in a state of scientific limbo. Maybe they are the oldest fossils on Earth, the remains of ancient iron oxidizing bacteria. Or maybe they’re chemical gardens, evidence of weird chemistry. Or maybe they’re something else entirely! Until more evidence is gathered, we’ll have to leave this mystery unsolved for now.
Summary:
If there’s any place that could have Earth’s oldest fossils, it’s Nuvvuagittuq, on the frigid tundra shores of north Quebec. 3.8 billion years ago, March 3rd on the Earth Calendar, this area was deep beneath the sea, next to volcanos and hot springs making an iron-rich soup. There was water, there was food, and there was almost certainly life here.
Whether there are any fossils left is still up for debate. Finding Earth’s earliest life is a tricky job, and evidence is still being gathered. I’ll be following this story closely and if there are any updates, you’ll be the first to know.
For now, it’s time to leave Canada behind. We’ve spent four episodes at Nuvvuagittuq, which have honestly left more questions than answers. Are these rocks actually 3.8 billion years old, or are they much older? Was this area a deep-sea trench, or something even weirder? Are there any fossils here or not? It’s time for some new stories.
In our imaginary submarine, we breach the surface of the sea. It’s night-time once again. The moon is much farther away now than the fist-sized orb we met in Season 1, but still twice as wide than we see it today. The surface is like a giant golf ball- no Man in the Moon just yet. Plenty of light to navigate to the next location.
Hmm, let’s check the Archean map. OK, looks like there’s a decent sized island just a few days away! Set a course for G…
For an instant, the map, the boat, the ocean is lit up like daylight. And in the next second, a dim darkness. There is no wind, no tsunami, no supersonic boom. But in the sky, a cloud of dust has enveloped the moon. As it slowly clears, an angry bleeding red eye is revealed, peering down on the world.
It looks like I spoke too soon. The Man in the Moon is just being born.